BACKGROUND: Ibrutinib is a BTK inhibitor approved for relapsed/refractory MCL. We evaluated the safety and efficacy of single-agent ibrutinib maintenance (I-M) following frontline chemo-immunotherapy for MCL in a multicenter phase II trial. Herein, we report preliminary results.
METHODS: Patients (Pts) received I-M 560 mg daily for up to 4 years after complete/partial response (CR/PR) to frontline chemo-immunotherapy (+/- autoSCT). The 3-year PFS rate was the primary endpoint. Secondary endpoints were PR to CR conversions, median OS and toxicity. After identifying a trackable clone using archived tissue obtained at diagnosis, minimal residual disease (MRD) was measured using NGS (detection resolution of 1 cell per million; clonoSEQ®; Adaptive Biotechnologies) on peripheral blood at 4 time-points: baseline (after induction but prior to starting I-M) and then at 1, 6 and 18-24 mo(s) after initiation of I-M.
RESULTS: 36 pts were enrolled between 7/2014-7/2018. Median age was 60 years (range 46-90). For induction, 17 (47%) received BR, 18 (50%) a cytarabine-containing regimen and 1 (3%) R-CHOP. Eighteen (50%) had autoSCT in CR1. Post-induction +/- autoSCT, 33 (92%) had a CR and 3 (8%) had a PR as best response respectively. One patient converted from PR to CR on I-M.
Median f/u was 34.6 months. 5 (14%) pts completed the 4-year I-M course and 15 (42%) remain on I-M (median 31 cycles, range 2-52). Sixteen (44%) pts discontinued I-M early due to: treatment related adverse events (TRAEs; n=12), second malignancies considered unrelated to I-M (n=2), PD (n=1) and death of unknown cause (n=1). Three pts died, 2 deaths deemed unrelated to I-M (aspiration pneumonia, 2nd malignancy) and 1 from unknown cause; 1 pt was lost to follow-up.
The most common grade ≥ 3 TRAEs during I-M were neutropenia, HTN, infection, bleeding and atrial fibrillation/flutter (Table 1). TRAEs led to permanent dose reductions in 9 (25%) pts, most commonly for neutropenia (n=3), and I-M discontinuation in 12 (33%), 6 for new onset atrial fibrillation/flutter, 1 each for myalgias, rash, pericardial effusion, mucositis, TIA, subdural hematoma/bleed. Notably, I-M was discontinued in the majority of pts with new onset atrial fibrillation/flutter though not typical of current practice.
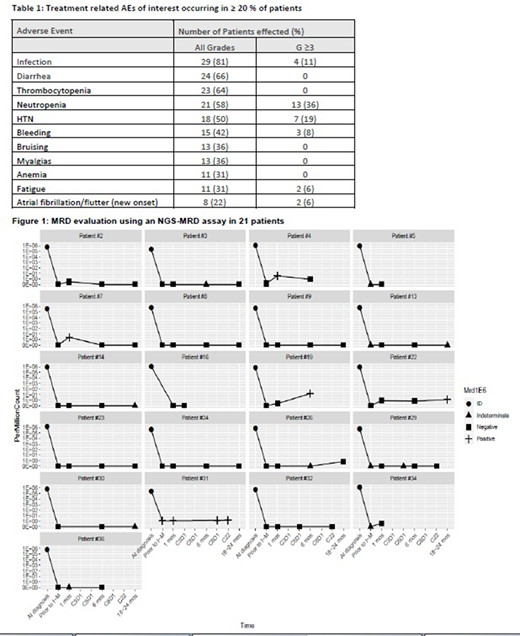
At the time of data cut-off, MRD was assessed in 21 of 36 pts at varying time points (Figure 1) with remaining samples not yet collected/analyzed. After induction but prior to I-M, 16 pts were confirmed MRD (-), 4 were MRD indeterminate and 1 was MRD (+). Four pts MRD (-) prior to I-M became MRD (+) with follow-up, 2 induced with hyperCVAD, 1 with BR and 1 with R-CHOP + autoSCT prior to I-M; 2 of these pts reverted back to MRD (-) status with ongoing I-M. Of the 2 pts with persistent MRD (+) disease, 1 had clinical progression and the other maintains radiographic CR. Post induction, all MRD indeterminate pts were MRD (-) when checked after 1 month on I-M. The pt who was MRD (+) after front-line treatment (Nordic regimen + autoSCT) remains MRD (+) after 30 cycles of I-M without clinical relapse. MRD correlations with PFS and OS have not yet been performed.
CONCLUSION: I-M 560 mg daily is feasible in pts with MCL after response to frontline chemo-immunotherapy. Grade ≥ 3 rates of atrial fibrillation/flutter are consistent with ibrutinib's known safety profile. NGS-based assessments demonstrate that most pts are MRD negative after intensive induction. Longer follow-up and correlation of MRD with PFS and OS are needed to determine the clinical relevance of I-M and MRD status.
Karmali:BeiGene: Speakers Bureau; AstraZeneca: Speakers Bureau; Karyopharm: Honoraria; Takeda: Research Funding; BMS/Celgene/Juno: Honoraria, Other, Research Funding, Speakers Bureau; Gilead/Kite: Honoraria, Other, Research Funding, Speakers Bureau. Stephens:Arqule: Research Funding; Innate: Consultancy; Juno: Research Funding; Acerta: Research Funding; MingSight: Research Funding; Janssen: Consultancy; Karyopharm: Consultancy, Research Funding; Beigene: Consultancy; Pharmacyclics: Consultancy; Gilead: Research Funding; Verastem: Research Funding. Winter:Norvartis: Consultancy, Other: DSMB; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Other: advisory board; Epizyme: Other: DSMB; Delta Fly Pharma: Consultancy; Amgen: Consultancy; CVS/Caremark: Consultancy; Ariad/Takeda: Consultancy; Merck: Membership on an entity's Board of Directors or advisory committees, Other: advisory board. Ma:Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; BeiGene: Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Research Funding; Bioverativ: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Juno: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Kite: Consultancy, Honoraria; TG Therapeutics: Research Funding. Petrich:AbbVie: Current equity holder in publicly-traded company; Daiichi-Sankyo: Current Employment. Hochberg:Intervention Insights: Consultancy; Leuko: Consultancy. Kuhr:Adaptive Biotechnologies: Current Employment. Gordon:Zylem Biosciences: Patents & Royalties: Patents, No Royalties. Pro:Verastem Oncology: Research Funding.
maintenance ibrutinib after frontline therapy in MCL
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal